“HPV-DNA tests are relatively new tools, and some countries are only beginning to implement them as part of their cervical cancer screening systems. WHO encourages and supports these changes,” said Dr Corbex. “In addition, DNA tests make it possible to redesign screening programmes to improve their quality, which is necessary on the way to eliminating cancer as a life-threatening disease in the WHO European Region and beyond.”
“Screening is not only the test itself, it is all that happens afterwards in cases where the woman is tested positive for cervical cancer. If further diagnostics and treatment components are not perfectly organized and not performed with high quality standards, advanced testing methods can be considered useless,” added Dr Corbex. In countries with effective cytology-based cervical cancer screening and treatment programmes, the mortality from cervical cancer has been reduced fivefold over the past 50 years. Yet, this approach has not been as successful in low- and middle-income countries. HPV DNA-based screening tests are more cost-efficient and are suitable for all settings, areas and countries. This is in line with the WHO European Programme of Work 2020–2025, which is based on the idea that health is a universal value, and no one should be left behind in access to health care.
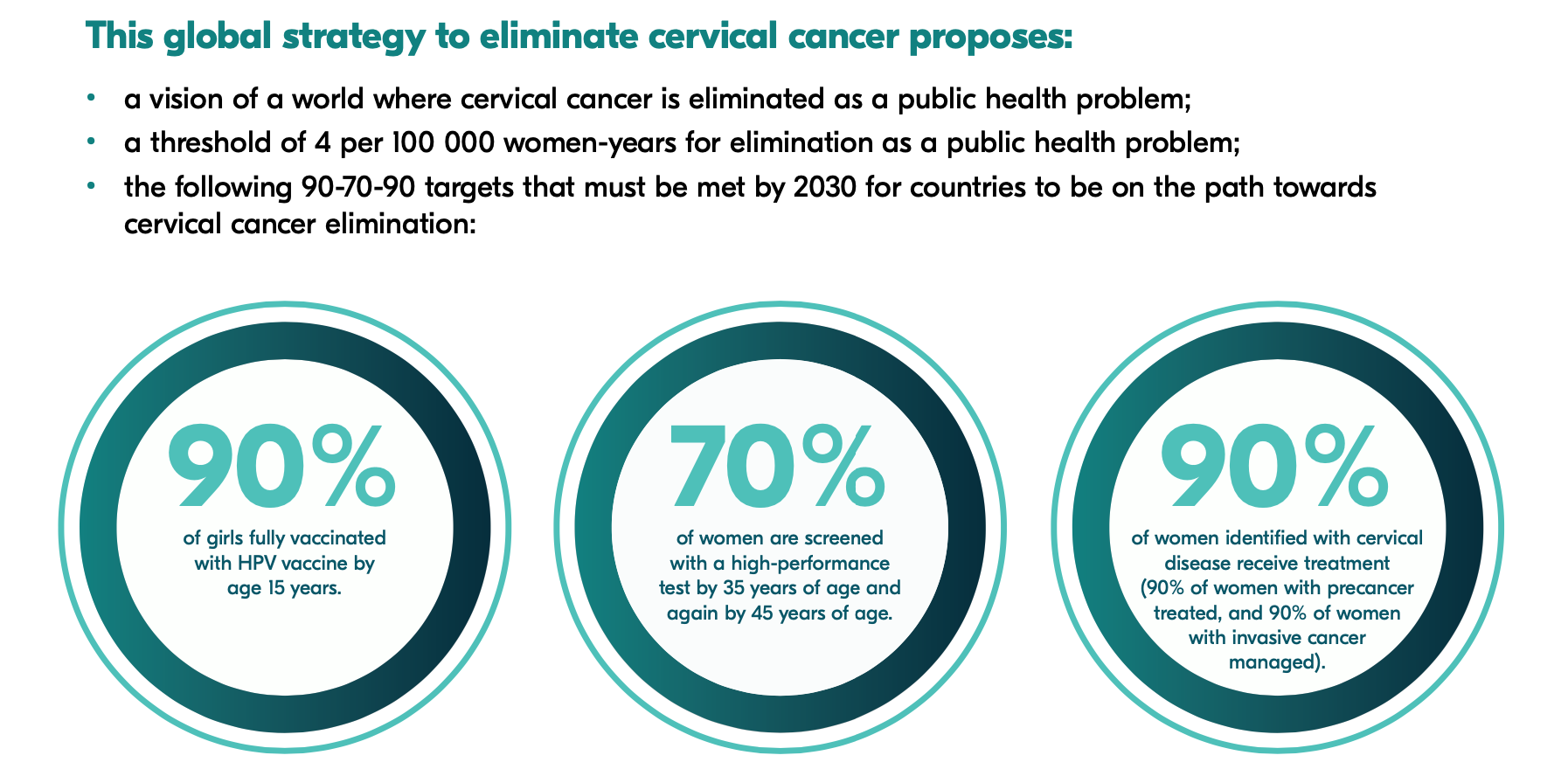
In 2020, WHO launched the Global Strategy to Accelerate the Elimination of Cervical Cancer. The goals of the strategy are, by 2030:
- To vaccinate 90% of eligible girls against HPV;
- To screen 70% of eligible women at least twice in their lifetimes.
- To effectively treat 90% of those with a positive screening test or a cervical lesion, including with palliative care when needed.